Executive Summary
Regulatory change monitoring in life sciences is becoming increasingly complex as global and regional health authorities issue a growing volume of guidance across multiple formats and jurisdictions. Agencies such as the FDA, EMA, MHRA, PMDA, and Health Canada, as well as regulators in emerging markets, release frequent, often unscheduled updates that affect submissions, labeling, quality systems, and post-approval obligations. Manual approaches, based on spreadsheets, alerts, and periodic reviews, are no longer sufficient to ensure timely interpretation, consistent impact assessment, or inspection readiness.
Generative AI (Gen AI) enables a more scalable and structured approach by automating the ingestion, classification, and summarization of regulatory content and assessing relevance against specific products, dossiers, and markets. When combined with retrieval mechanisms and rule-based filters, Gen AI can generate traceable impact assessments and support downstream updates to submissions and labeling. Integration with RIMS, eDMS, QMS, and labeling platforms is essential to move from awareness to execution and tracking. Successful adoption requires treating AI-enabled regulatory change monitoring as an operating model and architecture transformation rather than a point solution. Enterprises must define clear outcomes, establish a harmonized regulatory data backbone, embed human-in-the-loop governance, and implement incrementally through high-value use cases. Market solutions from providers such as RegASK, Clarivate, Freyr, and IQVIA illustrate how these capabilities are transitioning from pilots to core regulatory infrastructure. Over the next three to five years, AI-enabled regulatory change monitoring is expected to become a foundational capability across regulatory, quality, and safety operations.
Gen AI as an Enabler of Regulatory Change Monitoring
Regulatory change monitoring involves the continuous tracking and interpretation of updates issued by regulatory authorities, standards bodies, and guidance-setting organizations that directly affect product life cycle development, regulatory submissions, labeling governance, quality management systems, and post‑market surveillance activities. Historically, regulatory affairs teams have relied on the manual review of agency websites, email alerts, and third-party reports, resulting in high effort, delays, and inconsistent interpretation. Gen AI, built on large language models and retrieval-based architectures, enables automated ingestion and analysis of regulatory texts, improving the speed and consistency of regulatory intelligence while complementing, not replacing, expert judgment.
Key capabilities and benefits
-
- Automated detection of regulatory updates: Continuous monitoring of authoritative sources with relevance filtering by region, product type, and life cycle stage
- Summarization and impact analysis: Condensing complex regulatory language into structured, actionable insights aligned to specific products and dossiers
- Contextual relevance scoring: Mapping regulatory changes to internal submissions, SOPs, and historical authority interactions using semantic similarity
- Support for submissions and compliance: Assisting with updates to dossiers, labels, and internal documentation in response to new requirements
Key enablers
-
- Scalable ingestion and normalization of diverse regulatory content formats
- Semantic parsing of obligations, timelines, and references at the clause level
- Automated generation of impact summaries, internal change notices, and draft remediation actions for expert review
Technology Foundation for a Gen AI-Enabled Regulatory Change Monitoring Platform
An AI-enabled regulatory change monitoring platform is typically implemented as a layered technology stack that integrates external regulatory intelligence with internal submission, quality, and compliance systems. The architecture encompasses both technical and operational components, spanning data acquisition, content storage and indexing, artificial intelligence and analytics, and user-facing applications. Together, these elements support the continuous detection, interpretation, and operationalization of regulatory changes in a controlled and auditable manner.
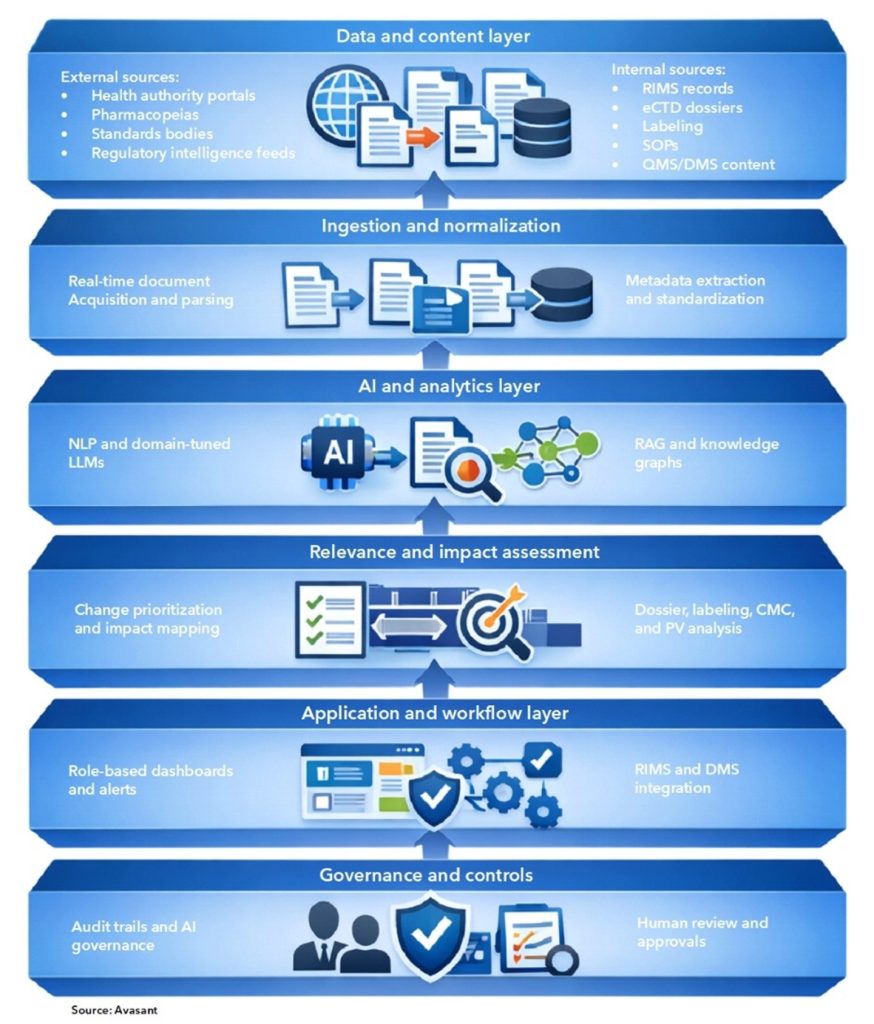
How Gen AI Works in Regulatory Change Monitoring
Gen AI-enabled regulatory change monitoring operates as a continuous, closed-loop process that moves from detection to action and learning. The workflow integrates automated intelligence gathering with expert oversight to ensure accuracy, relevance, and auditability.
-
- Detect and triage: Automated crawlers and connectors continuously scan predefined regulatory sources, including the FDA, EMA, ICH, CMDh, and national health authorities. Newly issued or updated documents are ingested as structured text. A Gen AI layer performs initial triage, prioritizing updates based on factors such as product class, therapeutic area, life cycle stage, jurisdiction, and historical query or inspection patterns.
- Interpret and summarize: Natural language processing and Gen AI models analyze documents to identify substantive changes, including new obligations, revised timelines, transition provisions, and cross-references. The system generates concise, structured summaries tailored to different regulatory roles, with traceability to specific clauses or paragraphs. Multilingual content is summarized in a common working language while preserving the original text for formal interpretation.
- Map to internal portfolio and submissions: Using ontologies, taxonomies, and semantic similarity, the platform links regulatory changes to internal products, dossiers, labeling, SOPs, and commitments. Outputs typically include lists of impacted CTD modules, labeling sections, clinical, CMC, and pharmacovigilance artifacts.
- Recommend and trigger actions: Gen AI proposes concrete actions, such as label updates, protocol amendments, or initiation of variations or supplements. Draft internal notifications and revised text can be generated for expert review. Integration with RIMS, QMS, and workflow tools enables task creation, approvals, and SLA tracking.
- Learn and refine: Human review outcomes, authority feedback, and inspection findings are captured to refine prioritization rules and model performance over time, reducing noise and improving relevance.
Enterprise Considerations for Implementing AI-Enabled Regulatory Change Monitoring
Enterprises should approach AI-enabled regulatory change monitoring as an operating model and architecture transformation rather than a point solution. The starting point is to define a clear vision and scope with measurable outcomes, such as reducing the time between regulatory publication and impact assessment, lowering the risk of missed changes, decreasing follow-up questions from health authorities, and improving inspection readiness. This vision should be translated into a target operating model that clarifies ownership across regulatory affairs, labeling, CMC, pharmacovigilance, and quality, along with defined service levels and escalation paths.
A reliable regulatory data backbone is critical. Organizations should consolidate external regulatory sources and harmonize internal systems, such as RIMS, eCTD repositories, labeling platforms, and QMS/DMS, to ensure AI models operate on consistent, structured inputs. The underlying architecture should be layered and modular, integrating with existing platforms rather than duplicating them. Initial implementation should focus on two to three high-value use cases, such as monitoring guideline changes in a priority therapy area or generating product-specific label impact alerts, to demonstrate tangible value.
Governance must be embedded from the outset. This includes human-in-the-loop review, documented decision logs, full traceability to source documents, and a risk-based validation approach aligned with computerized system validation and assurance practices. Change management is equally important: users must be trained, feedback actively captured, and workflows refined iteratively so AI outputs become part of routine regulatory work. Finally, enterprises should adopt a deliberate vendor and ecosystem strategy that balances core regulatory platforms, cloud infrastructure, and specialized AI tools while minimizing redundancy and long-term dependency.
Market Solutions and Real‑World Implementations
Numerous vendors and industry initiatives are actively implementing AI-enabled regulatory change monitoring, providing practical examples of how the technology can be operationalized. These RegTech and AI-driven regulatory intelligence solutions highlight key architectural patterns and operating models in practice.
| Solution/Platform |
Technology approach |
Capabilities |
| RegASK (RegGenius)[i] |
NLP, machine learning, and Gen AI models trained on regulatory corpora and automated ingestion of global regulatory sources with expert-in-the-loop validation |
Continuous global regulatory change monitoring, summarized and prioritized updates, portfolio- and market-specific impact assessment, and risk and trend identification |
| Clarivate — Cortellis Regulatory Assistant[ii] |
Structured regulatory intelligence database augmented with AI-driven contextual search, summarization, and guidance interpretation |
Centralized regulatory intelligence, faster navigation of complex guidance, and support for submission planning and regulatory strategy alignment |
| Freyr — freya fusion (freya.intelligence)[iii] |
AI analytics combined with expert-curated regulatory data; GenAI assistants for query response and scenario analysis |
Always-on monitoring and alerts, impact insights across markets, regulatory outcome scenarios, and support for global submission readiness |
| IQVIA — AI-enabled regulatory services[iv] |
Large-scale data ingestion, AI-driven text analytics, and cross-market comparison models integrated with regulatory datasets |
Early detection of regulatory changes, impact analysis across global portfolios, improved submission alignment, and reduced manual monitoring effort |
| IONI AI — Life sciences regulatory monitoring[v] |
AI-powered ingestion and classification of regulatory updates and Gen AI-based summarization and alerting |
Timely identification of regulatory changes, compliance-focused alerts, and actionable insights for regulatory and quality teams |
| SyneticX[vi] |
AI-driven data ingestion with analytics dashboards and alerting mechanisms |
Unified regulatory information view, real-time alerts, and tracking of regulatory changes and submission-related activities |
| HARMAN i-QARA[vii] |
Gen AI-enabled document processing, regulatory ingestion, and mapping to internal QMS and regulatory artifacts |
Automated compliance monitoring, linkage of regulations to quality and regulatory processes, and generation of audit-ready documentation |
| Regology — Reggi[viii] |
Automated aggregation of legal and regulatory content and Gen AI-assisted drafting of compliance responses and query answers |
Centralized monitoring of regulatory changes, AI-assisted interpretation and response generation, and faster compliance decision-making |
| Visualping (generalist example)[ix] |
AI-based web change detection and notification |
Fundamental change detection and alerting and adaptable monitoring patterns for regulatory content sources |
Industry pilots and studies show that AI-enabled monitoring can identify regulatory changes more quickly, summarize extensive documents efficiently, and accelerate impact assessment compared with manual methods. These implementations indicate a trend toward integrated platforms that merge curated regulatory content, AI-driven analysis, and seamless connectivity with regulatory and quality systems, rather than function as isolated intelligence portals.
Conclusion
AI-enabled regulatory change monitoring is reshaping regulatory intelligence by moving organizations away from periodic, manually driven reviews toward a continuous, portfolio-aware operating model. From Avasant’s perspective, this shift is less about adopting a new tool and more about redesigning how regulatory intelligence is produced, governed, and consumed across the enterprise. Effective implementations combine Gen AI with curated regulatory content, tight integration with RIMS, eDMS, labeling, and quality systems, and clearly defined operating ownership across regulatory, CMC, pharmacovigilance, and quality teams. Automating the detection, summarization, and contextual mapping of regulatory updates materially reduces the effort required to assess impact and initiate submission or labeling changes. However, value is realized only when these capabilities are embedded within a structured architecture that supports traceability, inspection readiness, and human-in-the-loop validation. Enterprises that succeed treat Gen AI as an enabler of consistency and speed, not a substitute for regulatory judgment. Continuous monitoring, LLM-driven interpretation, and disciplined governance, supported by measurable KPIs, allow regulatory teams to shift focus from repetitive surveillance to higher-value analysis and decision-making. As regulatory requirements continue to expand across products, regions, and emerging digital and AI-enabled therapies, organizations that institutionalize Gen AI within regulatory operations will be better positioned to deliver predictable submissions, reduce compliance risk, and respond with confidence to regulatory change.
References
[i] https://regask.com/reggenius-pr/
[ii] https://clarivate.com/life-sciences-healthcare/research-development/regulatory-compliance-intelligence/regulatory-intelligence-solutions/
[iii] https://www.freyafusion.com/products/freya-intelligence
[iv] https://www.iqvia.com/solutions/safety-regulatory-compliance/regulatory-compliance/global-regulatory-affairs-services
[v] https://ioni.ai/life-sciences-pharma-regulatory-software
[vi] https://www.syneticx.com/Regulatory-Intelligence
[vii] https://services.harman.com/lifesciences/qara
[viii] https://regology.com/reggi
[ix] https://visualping.io/
Abbreviations
AI — Artificial intelligence
API — Application programming interface
CMC — Chemistry, manufacturing, and controls
CMDh — Coordination Group for Mutual Recognition and Decentralised Procedures – Human
CTD — Common technical document
DMS — Document management system
eCTD — Electronic common technical document
eDMS — Electronic document management system
EMA — European Medicines Agency
FDA — Food and Drug Administration
Gen AI — Generative artificial intelligence
ICH — International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
LLM — Large language model
MHRA — Medicines and Healthcare products Regulatory Agency
NLP — Natural language processing
PMDA — Pharmaceuticals and Medical Devices Agency
QMS — Quality management system
RAG — Retrieval‑augmented generation
RIMS — Regulatory information management system
SLA — Service-level agreement
SOP — Standard operating procedure
By Eratha Poongkuntran, Associate Director, Samkit Jain, Lead Analyst


