This report identifies key demand-side trends in the clinical data management business process transformation (BPT) space, helping enterprises fine-tune their clinical operations. It provides an overview of key observations and business challenges that Avasant considers important to highlight in the clinical data management outsourcing space.
Why read this Market Insights?
Clinical data management is changing in response to more complex trial designs, higher R&D costs, and evolving regulatory requirements. The field is moving toward integrated approaches that use electronic data capture, risk-based quality management, and real-time analytics. Increasing use of AI, automation, and standardized data models is shaping how data is collected, reviewed, and prepared for submission. The emphasis is shifting from manual processes to more systematic methods that improve data accuracy, shorten timelines between last subject visit and database lock, and support large-scale, multiregional trials.
The Clinical Data Management Business Process Transformation 2025 Market Insights™ provides a view into important market trends and developments, helping build a granular understanding of the clinical data management business process transformation ecosystem.
Methodology
The market insights presented in this report are based on our ongoing interactions with enterprise CXOs and other key executives, targeted discussions with service providers, subject matter experts, and Avasant fellows, analyst insights derived from primary and secondary research, and lessons learned from consulting engagements.
Table of contents
About the Clinical Data Management Services Business Process Transformation 2025 Market Insights report (Page 3)
Executive summary (Pages 4–7)
-
- Definition and scope of clinical data management business process transformation
- Key clinical data management market trends shaping the industry
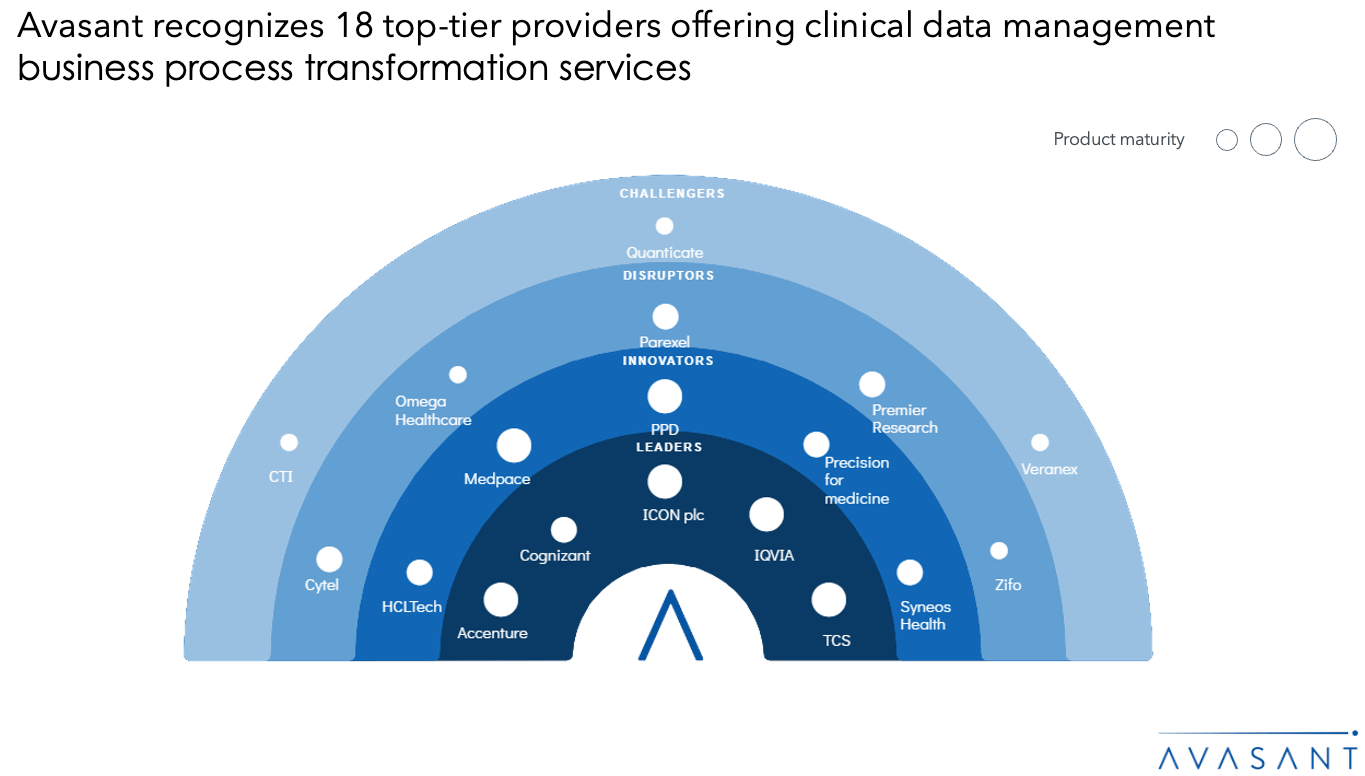
- Avasant recognizes 18 top-tier providers offering clinical data management business process transformation services
Demand-side trends (Pages 8–13)
-
- Patient-centric data capture is revolutionizing clinical data management through advanced platforms, remote monitoring, and diverse digital sources.
- Real-world data was used in 65% clinical trials through harmonized formats, AI tools, and regulatory support for eligibility and monitoring.
- By 2024, 96% of trials included at least one risk-based quality component, with sponsors reallocating CDM budgets toward automation and AI-based oversight.
- CDM is consolidating around enterprise platforms through repeatable sponsor–CRO partnerships and integrated trial delivery models.
- Recent regulatory updates are altering how clinical data is managed, shifting expectations around AI use, data exchange, and compliance standards.
Key contacts (Pages 14)
Read the Research Byte based on this report. Please refer to Avasant’s Clinical Data Management Business Process Transformation 2025 RadarView™ for detailed insights on service providers and supply-side trends.