Life sciences organizations operate in some of the most stringent regulatory environments globally, governed by frameworks such as the FDA, EMA, cGMP, and GxP, as well as data integrity standards. Maintaining compliance is essential not only for regulatory approval but also for ensuring product quality, patient safety, and corporate reputation. Traditional compliance processes are often reactive, heavily manual, and data-fragmented—making it difficult to anticipate risks before they result in violations. Predictive identification of compliance risks, enabled by artificial intelligence (AI), offers a significant shift in approach. By leveraging historical audit and operational data, AI models can identify patterns and signals of potential noncompliance, enabling proactive intervention and continuous quality improvement.

Recent data highlights the scale and impact of compliance lapses across the life sciences sector. In 2024, the FDA issued over 560 Form 483 observations to drug manufacturers in 2024—up sharply from 215 in 2021—with nearly 17% linked to deficiencies in stability programs[i]. A study published in JAMA found that 85% of major pharmaceutical firms incurred penalties between 2003 and 2016, amounting to more than $33 billion, primarily for off-label promotion, pricing violations, and kickbacks[ii]. Broader analyses estimate that since 1991, the industry has paid over $62 billion in fines across nearly 500 cases[iii]. Individual examples include Endo Health Solutions’ $1.5 billion criminal settlement in the opioid case[iv] and Novo Nordisk’s reprimand in the UK for failure to disclose £7.8 million in payments[v]. These incidents underscore recurring issues in data integrity, documentation, and transparency, often resulting in regulatory sanctions, operational disruptions, and reputational damage. The upward trend in FDA observations and global enforcement actions illustrates increasing scrutiny and the growing financial stakes associated with noncompliance for life sciences organizations.
Compliance Challenges in Life Sciences
Despite mature quality systems, compliance in life sciences remains vulnerable to human error, fragmented data, and rapidly evolving regulations. The sector faces challenges in maintaining audit readiness, managing third-party risks, and ensuring continuous alignment with standards like FDA, EMA, and GxP. These systemic gaps underscore the need for intelligent, data-driven compliance management. Compliance management in life sciences faces several persistent and systemic challenges:
- Regulatory complexity: Organizations must comply with diverse and frequently changing global regulations (FDA, EMA, HIPAA, GDPR, and so on). Constant updates to standards and guidelines require continuous training, process adaptation, and documentation.
- Data volume and fragmentation: Compliance-related data resides across multiple systems—quality management systems (QMS), laboratory information management systems (LIMS), ERP, CRM, and document repositories—making it difficult to achieve unified visibility.
- Manual and reactive processes: Reliance on manual reviews, periodic audits, and retrospective investigations limits the ability to detect early warning signs.
- Resource constraints: Lean compliance teams struggle to manage large data volumes, especially during audits, product launches, and mergers and acquisitions.
- Third-party and supplier risks: Ensuring GxP compliance among suppliers, contract manufacturers, and contract research organizations (CROs) adds layers of complexity and risk.
- Audit readiness: Gaps in data integrity, incomplete validation records, or inconsistent documentation can lead to regulatory citations and product approval delays.
How AI Can Address Compliance Risks
AI technologies can transform compliance management by analyzing large volumes of structured and unstructured data to identify patterns associated with past compliance deviations. Rather than waiting for violations to surface, AI systems can continuously monitor data flows to flag potential risks early. Its key contributions include:
-
- Predictive risk identification: Machine learning (ML) models trained on historical audit and compliance data can detect patterns that precede noncompliance incidents, allowing for preventive actions.
- Automation of monitoring and auditing: AI-powered tools can review vast data streams in real time—across manufacturing, clinical, and financial processes—automatically flagging anomalies linked to potential breaches.
- Reduction in manual workload: Routine tasks such as document reviews, cross-checking data, or compiling reports can be automated, freeing compliance teams for strategic oversight.
- Dynamic risk scoring: AI continuously recalculates risk scores as new data becomes available, enabling real-time monitoring of high-risk activities (for example, healthcare professional (HCP) engagements or supplier transactions).
- Enhanced audit readiness: Automated data consolidation and intelligent document management streamline audit preparation and improve transparency for regulatory inspections.
- Contextual decision support: AI systems can classify deviations, suggest corrective measures, and surface related precedents to support compliance officers in decision-making.
Key Sources of Compliance Data
Building effective AI compliance models requires a wide range of high-quality data from across the product life cycle. Audit logs, manufacturing records, regulatory submissions, and third-party data collectively provide insights into risk trends. Ensuring data integrity, completeness, and traceability is essential for generating reliable predictions. Predictive models depend on access to comprehensive and reliable data. In the life sciences industry, relevant data sources include:
| Data sources/systems |
Typical data provided |
| QMS |
Deviation reports, corrective and preventive action (CAPAs), change controls, complaints handling, and audit trails |
| Audit and inspection systems |
Internal/external audit reports, FDA 483, warning letters, inspection findings, and remediation actions |
| Manufacturing execution/batch records |
Batch production records, equipment logs, calibration, maintenance history, environmental monitoring, and materials traceability |
| LIMS |
Sample life cycle data, test results, stability studies, validation protocols, and audit trails |
| Document management systems (DMSs) |
SOP repositories, controlled documents, version control, and revision/approval logs |
| Training and HR systems |
Employee qualifications, training completion, and competency assessments |
| Pharmacovigilance and safety systems |
Adverse event reports, signal detection outputs, and regulatory submission logs |
| Regulatory filing platforms |
Submission dossiers (eCTD), agency correspondence, and approval/denial documentation |
| Commercial transparency and expense reporting tools |
Transfer of value reports, HCP/HCO interaction logs, and CMS Open Payments data |
| Supplier/third-party management |
Vendor qualification, performance scores, audit results, and incident management logs |
| Real-world/post-market surveillance |
Product complaint databases, device tracking (UDI), and post-market surveillance reports |
| ERP systems |
Procurement history, inventory, finance records, and supply chain traceability |
| IT security and access logs |
User authentication, system changes, and cybersecurity incidents |
| Master data management |
Harmonized HCP/HCO, product, and customer reference data |
Note: This is not an exhaustive list.
AI Technology Stack for Compliance Risk Prediction
The technical foundation of an AI-driven compliance platform in life sciences is built on a secure data infrastructure that centralizes information from multiple operational systems, including quality management, laboratory, manufacturing, document management, and regulatory submission systems. This infrastructure typically uses cloud-based data storage following compliance requirements such as 21 CFR Part 11 and GxP standards. Data ingestion pipelines extract, transform, and load data uniformly, ensuring consistent formats, deduplication, and integrity. A governance layer enforces security policies, access control, audit trails, and data lineage to meet regulatory scrutiny requirements. Encryption protects data confidentiality both in transit and at rest, while identity and access management systems regulate user permissions and activities.
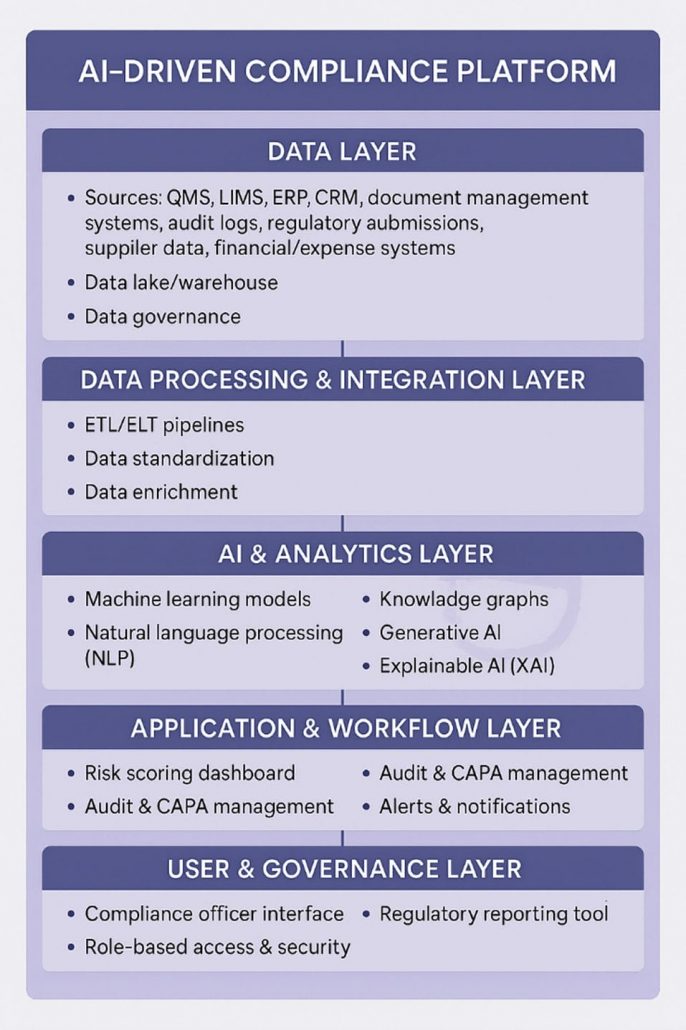
On top of this foundation, AI models operate within a controlled environment that enables scalability and reproducibility. ML algorithms analyze historical compliance and operational data to identify risks and anomalies, while NLP tools process unstructured documents such as SOPs and audit reports. Knowledge graphs offer contextual connections between entities such as products, processes, and suppliers, improving risk interpretation. Generative AI automates preparation of corrective action reports and audit summaries. Explainability modules are incorporated to provide transparent insights into AI decisions for auditing purposes. The platform layers in application interfaces such as dashboards, alerting systems, and workflow tools allow compliance teams to review predictive insights, manage corrective actions, and prepare regulatory submissions. All platform changes go through formal change controls, and environments are continuously monitored for compliance to ensure regulatory requirements are sustained over time.
AI Compliance Solutions in the Market
Several vendors now offer AI-enabled compliance platforms designed specifically for life sciences. These solutions integrate predictive analytics, automation, and natural language tools within existing QMS or ERP systems. They enable organizations to streamline compliance workflows, enhance risk visibility, and maintain regulatory readiness more effectively.
| Solution |
Core features |
Key applications |
| MasterControl |
AI-enhanced quality event detection, no-code process design, and automated audit trails |
Quality event management, CAPA, and audit readiness |
| Veeva Vault QMS |
Integrated AI agents for document intelligence and secure data access within workflows |
Document control, deviation management, and audit preparation |
| Sparta TrackWise Digital |
AI-driven trend detection, auto-summarization, and categorization using NLP |
CAPA, deviation management, and risk-based decision-making |
| AuditComply |
AI-supported audit planning, risk-based issue classification, and real-time dashboards |
Audit management, supplier quality, and compliance monitoring |
| MetricStream |
AI/ML-powered issue classification, duplication detection, and action plan recommendations |
Issue and remediation management and GRC automation |
| IBM watsonx |
Governance accelerators for AI compliance, regulatory framework mapping, and risk management |
AI governance, regulatory compliance, and model monitoring |
| SAS Viya |
Trustworthy AI capabilities, including bias detection, explainability, and audit trails |
AI model governance, compliance auditing, and risk mitigation |
| Deloitte & IQVIA |
Proprietary AI compliance dashboards leveraging historical data |
Compliance monitoring, risk assessment, and regulatory strategy |
Note: This is not an exhaustive list.
Most leading platforms emphasize integration with enterprise systems (ERP, CRM, QMS, and so on) and compliance with key standards (FDA 21 CFR Part 11, EMA, ISO, and so on).
Conclusion
To remain competitive and compliant in an increasingly complex regulatory landscape, Avasant recommends that life sciences organizations transition from reactive to proactive AI-driven compliance models to meet evolving global regulations and enhance operational resilience. Predictive analytics enables early detection of compliance risks, safeguarding product quality, ensuring patient safety, and reducing the cost of noncompliance. A unified, compliant data infrastructure integrating QMS, LIMS, ERP, and audit systems is essential to ensure data integrity and real-time visibility. By leveraging AI technologies such as ML, NLP, and knowledge graphs, firms can automate monitoring, detect anomalies, and strengthen audit readiness, while embedding governance, explainability, and ethical AI principles to maintain regulatory trust. We believe that implementing AI compliance tools in critical areas such as deviation management, batch record review, and supplier oversight can showcase the tools value and scalability. Success in these initiatives relies on cross-functional collaboration, ongoing model validation, and strict adherence to GxP and 21 CFR Part 11 standards. By partnering with validated AI solution providers, enterprises can accelerate implementation, mitigate risks, and turn compliance into a strategic advantage. This approach will enhance efficiency, boost regulatory confidence, and support long-term business sustainability.
References
[i] https://www.gmp-compliance.org/gmp-news/fda-483s-due-to-deficiencies-in-the-stability-program-evaluation-of-fiscal-year-2024
[ii] https://jamanetwork.com/journals/jama/fullarticle/2772953
[iii] https://www.citizen.org/news/persistent-misconduct-forces-pharmaceutical-manufacturers-to-pay-62-3-billion-in-penalties/
[iv] https://oig.hhs.gov/fraud/enforcement/opioid-manufacturer-endo-health-solutions-inc-ordered-to-pay-1536b-in-criminal-fines-and-forfeiture-for-distributing-misbranded-opioid-medication/
[v] https://www.pmcpa.org.uk/cases/advertised-sanctions/public-reprimand-for-novo-nordisk-auth38471123/
Abbreviations
FDA – US Food and Drug Administration
EMA – European Medicines Agency
cGMP – current Good Manufacturing Practice
GxP – Good x Practice (The “x” represents the various areas it can be applied to. It is a collective term for quality and compliance standards such as Good Manufacturing Practice, Good Clinical Practice, and Good Laboratory Practice.)
By Eratha Poongkuntran, Associate Director, Avasant



